Immune Signals in Blood: A New Frontier for Predicting Immunotherapy Side Effects
The recent breakthrough achieved by researchers at UT Southwestern has sparked a renewed conversation about the future of cancer immunotherapy and the possibility of more personalized, safer treatment options. This study, which identifies early immune signals from the blood that may predict adverse reactions to immunotherapy, stands as a promising step forward in the effort to tailor cancer care to each patient’s unique biology.
As cancer immunotherapy continues to emerge as a key component in modern oncological treatments, its promising potential is accompanied by an equally challenging set of issues. The immune system, when supercharged to attack malignant cells, sometimes inadvertently harms healthy tissues, leading to troublesome side effects. Recognizing these “tricky parts” early can help clinicians figure a path toward mitigating these problems, ensuring the benefits of immunotherapy far outweigh the risks.
Understanding Immune Checkpoint Inhibitors and Their Hidden Complexities
Immune checkpoint inhibitors have dramatically altered how we approach cancer treatment. These drugs work by enhancing the body’s natural defense mechanisms, enabling immune cells to better recognize and destroy cancer cells. However, this escalation of immune response may also lead to a series of “tangled issues,” including damage to non-cancerous tissues.
In over half of the patients who receive immunotherapy, side effects appear that are not only challenging to predict but often very “complicated pieces” to diagnose. When a patient experiences reactions from these treatments, it can lead to an intimidating situation where doctors must balance treatment effectiveness against potential risks. The latest study introduces a method to dig into the hidden pieces within the blood, helping to highlight early warning signs of such adverse effects.
Key Biomarker Findings and Their Implications
At the heart of the breakthrough is a multi-omic biomarker analysis, which is a comprehensive process that examines various biological molecules to detect pre-existing inflammatory states. These early immune signals include:
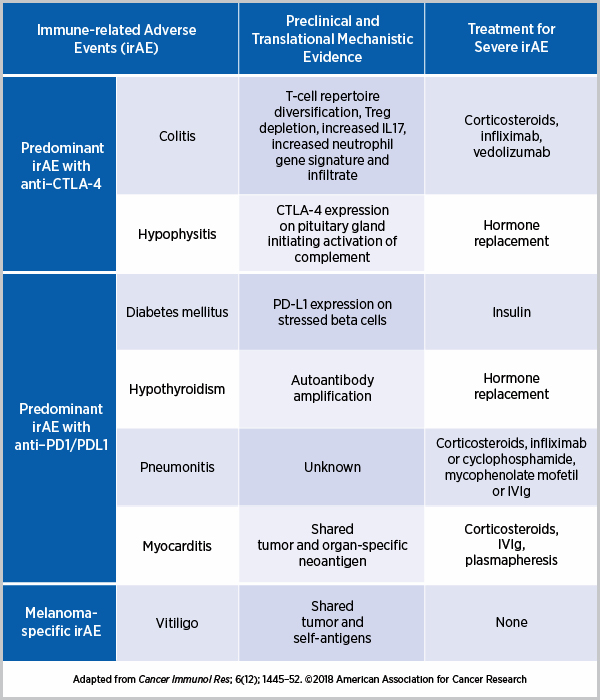
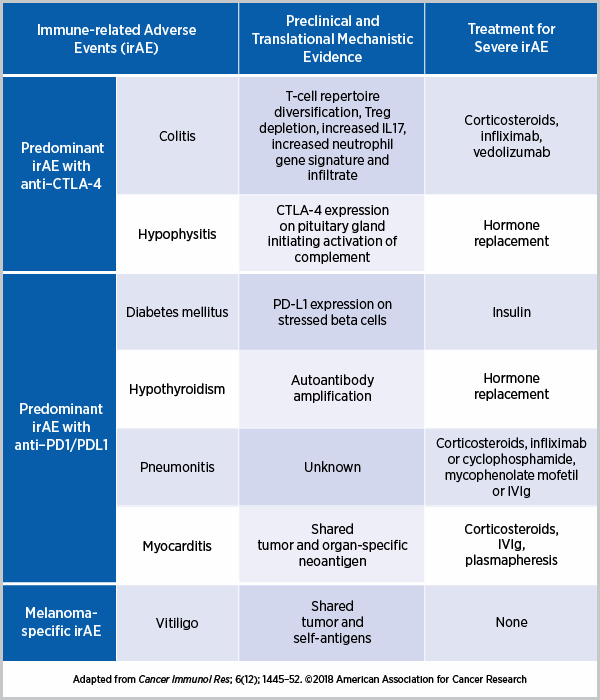
- Elevated Levels of Antibody-Producing Cells and Autoantibodies: The study observed that when antibody-producing cells are present in high numbers, and autoantibodies are detected, patients are more likely to develop side effects. These components are vital indicators that the body might be primed for an unintended immune response.
- Enhanced Activity of Inflammatory Molecules: An increase in inflammatory molecules, such as interferon-gamma, signals that the body might be responding to both cancer cells and healthy tissues. This dual role can be foreboding for patients likely to experience adverse reactions.
- Heightened Signals from Tumor Necrosis Factor (TNF): Elevated TNF levels also serve as a flag, suggesting that once the treatment begins, there may be a higher probability of side effects manifesting.
These findings create a foundation for future tests that can predict and potentially prevent harmful side effects before they emerge during treatment. As clinicians begin to explore these biomarkers further, there is hope that by understanding the subtle parts of the immune response, personalized care strategies can be developed, minimizing risks while harnessing the full power of immunotherapeutic drugs.
From Research to Real-World Impact: The Promise of Personalized Cancer Care
One of the most encouraging aspects of this study is its potential to shift how oncologists manage immunotherapy treatments. In the not-so-distant future, doctors may be able to perform a simple blood test to identify patients who carry these risky biomarker profiles, thereby enabling the customization of treatment plans from the very beginning.
Personalized treatment approaches are not just about administering the right drug at the right dose; they also involve identifying patients who might be particularly vulnerable to negative reactions. By pinpointing those with an already heightened proinflammatory state, physicians can proactively implement safety measures, adjust dosing, or even consider alternative therapies. This level of personalization is super important, as it provides individual patients with a treatment pathway that considers both the supercharged potential of immunotherapy and its possible pitfalls.
Challenges in Implementing Biomarker-Based Screening
While the prospect of using biomarkers for tailoring treatments is exciting, several challenges remain. First, replicating these findings across larger and more diverse patient populations is essential. Current studies conducted at leading institutions such as UT Southwestern and Parkland Health have laid the groundwork, yet more research is needed to confirm these results in different environments and among various demographic groups.
Moreover, establishing standardized protocols for biomarker testing may be a nerve-racking process initially. The variability in lab techniques and patient responses means that translating these findings into everyday clinical practice is a task full of fine points that require careful calibration. Nonetheless, the ultimate goal remains clear: instill confidence in clinicians and patients by predicting, diagnosing, and managing immune-related side effects more effectively.
Exploring the Pre-Treatment Immune Landscape
The study underscores the potential of multi-omic analyses in revealing a “clinically silent but proinflammatory state” that may exist even before treatment begins. Through an in-depth examination of blood samples collected from patients both before and after immunotherapy, researchers were able to detect subtle yet significant variations in immune activity.
These early signals are crucial because they indicate that, even in the absence of overt clinical symptoms, the patient’s immune system might already be primed for a vigorous response. Detecting these early changes could be the key to figuring a path through the inevitable twists and turns of cancer treatment, enabling more timely interventions that prevent severe side effects from escalating.
Decoding the Inflammatory Markers
An essential aspect of this study is the detailed evaluation of various inflammatory markers. Some of the critical players that were measured include:
- Interferon-Gamma: Known for its role in the immune response, an increased presence of interferon-gamma suggests that the body is preparing for an immune reaction that could inadvertently target healthy tissues.
- TNF (Tumor Necrosis Factor): Often involved in systemic inflammation, elevated TNF levels imply that the immune system is on high alert, which could lead to damaging side effects if not controlled.
- Autoantibodies: These proteins, which target the body’s own tissues, are key indicators that the immune system may be misfiring, setting the stage for adverse reactions once treatment commences.
In order to get a closer look at these markers, future studies may utilize advanced profiling techniques that combine genetic, molecular, and clinical data. Such integrative analyses could pave the way for more precise risk assessment tools, ultimately reducing the occurrence of complications and improving patient outcomes.
Personal Stories and the Broader Implications for the Cancer Community
Beyond the scientific details, it is important to consider the real-world implications of these findings for patients and their families. For many cancer patients, the journey through treatment is already overwhelming. The potential to face life-threatening side effects from immunotherapy only adds extra layers of worry. However, this research is a welcome glimmer of hope, as it suggests that one day, personalized assessments may spare patients from unexpected complications and allow them to focus their energy on beating cancer.
Imagine a scenario where, before starting immunotherapy, a patient undergoes a targeted blood test that reveals their specific risk profile. If the test indicates a heightened proinflammatory state, the medical team can adjust the treatment regimen appropriately or take preventative measures. This proactive approach not only supports the patient’s physical well-being but also helps alleviate the mental and emotional burdens associated with cancer treatment.
In addition to clinical benefits, such advancements hold promise in reducing the broader healthcare costs associated with managing adverse reactions. When side effects are prevented or mitigated early, patients are less likely to require extensive hospital stays or expensive emergency interventions, benefiting both the individual and the healthcare system at large.
Community Outreach and Education: Building Awareness About Biomarkers
For the community at large, the evolving landscape of personalized cancer care necessitates a renewed focus on education and awareness. Patients, caregivers, and medical professionals alike must be informed about the potential for biomarkers to predict treatment complications. Educational initiatives that translate scientific findings into practical information will be key in fostering trust in these new diagnostic tools.
Public health campaigns could, for example, include seminars, online resources, and interactive Q&A sessions that explain:
- What biomarkers are and how they function in the context of cancer treatment
- Why early detection of a proinflammatory state is critical
- How personalized treatment plans can reduce the risk of severe side effects
- The importance of ongoing research in refining these predictive tests
By taking these small steps toward public education, healthcare providers can demystify the process, allowing patients to feel more empowered and informed as they take their treatment journeys.
The Role of Institutional Research and Collaboration in Advancing Patient Care
The progress achieved by UT Southwestern and its partners is a testament to the power of institutional collaboration. By drawing on extensive patient registries, biospecimen collections, and years of accumulated clinical data, researchers have been able to piece together a more complete picture of how the immune system reacts during cancer treatment.
This study is the result of a long-standing commitment to integrating clinical practice with advanced research. It leverages data from over 800 patients treated at both UT Southwestern and Parkland Health, offering a broad snapshot of how varied immune responses can be across different demographic groups and treatment settings.
The collaborative nature of this research—spanning multiple departments, specialties, and even partnering institutions—illustrates how tackling the “little details” of a patient’s reaction to treatment requires a concerted, multi-disciplinary effort. From oncologists and pathologists to public health experts and immunologists, each professional brings a unique perspective that enriches the overall understanding of immunotherapy’s challenges and potential solutions.
Institutional Registries: A Treasure Trove of Clinical Insights
One key to this progress has been the establishment of robust patient registries. These databases compile detailed information on patient outcomes, treatment responses, and molecular profiles. They serve as invaluable resources, enabling researchers to:
- Identify recurring patterns in immune signal changes
- Spot early indicators of potential complications
- Track long-term outcomes relative to initial biomarker profiles
- Develop predictive models that can be refined with ongoing research
As these registries grow and evolve, they will continue to offer key insights into the “hidden complexities” of cancer treatment and help shape future strategies for early detection and intervention. Furthermore, as more institutions participate, the data pool will become even richer, potentially highlighting subtle distinctions that are critical for fine-tuning treatment protocols.
Balancing the Promises and Challenges of Immunotherapy
It is important to keep in mind that while the findings of this study represent a breakthrough, they are only one piece of a much larger puzzle. Immunotherapy has already transformed the treatment landscape for many forms of cancer, offering hope to thousands of patients with advanced stages of the disease. Yet, the journey from laboratory discovery to routine clinical application is full of twists and turns, and several challenges remain.
One major challenge is the inherent variability in patients’ immune profiles. What works as a predictive marker in one group might not hold the same significance in another, complicating efforts to create a universal standard for risk assessment. This reality makes it crucial for future research to be conducted on larger, more diverse populations. Only then can clinicians stitch together a comprehensive and reliable framework for predicting immunotherapy side effects.
In addition, the process of implementing biomarker testing on a wide scale will require extensive training, modifications in clinical practice, and a refined understanding of how these tests should inform treatment decisions. It is a process that might appear overwhelming at first, but with persistence and collaboration across the medical community, the benefits are likely to outweigh the early challenges.
Overcoming the Nerve-Racking Aspects of Change in Medical Practice
For many healthcare professionals, the idea of integrating new diagnostic tests into routine practice can feel off-putting. There are concerns about the reliability of these tests, the extra time and resources needed for analysis, and the possibility of misinterpreting results. However, as with any innovation in medicine, the initial bumps along the road are part of the process of improvement.
To ease this transition, institutions might consider:
- Implementing targeted training sessions that help clinicians understand how to interpret biomarker data
- Developing clear clinical guidelines that integrate biomarker testing into treatment protocols
- Paving the way for cross-disciplinary collaboration where experts assist in the decision-making process
- Investing in advanced diagnostic equipment and streamlined data analysis tools
With these measures in place, the community can begin to see biomarker testing not as an intimidating hurdle but as a key, enabling tool in the fight against cancer. The potential payoff—a more personalized, effective, and safe treatment plan for patients—is well worth these initial adaptations.
Looking Ahead: Future Directions in Cancer Immunotherapy Research
While much has been accomplished, the path forward is filled with opportunities as well as challenges. Researchers continue to work through the subtle parts of immune system behavior in the context of cancer treatments, aiming to further refine predictive tools and develop strategies that mitigate side effects. The continuous evolution of this field is super important because it holds the promise of transforming cancer care for generations to come.
Future research initiatives will likely focus on:
- Expanding patient cohorts to include a broader demographic, ensuring that findings are applicable across different populations
- Incorporating more detailed genetic and molecular profiling to unveil hidden complexities within immune responses
- Establishing multi-center collaborations that pool data and expertise, which can help validate early findings
- Developing standardized clinical tests that can be easily integrated into everyday patient care
These steps are not only about improving the safety of immunotherapy—they represent a shift towards a future where treatment is as personalized as the patients who receive it. As research continues, we anticipate that more precise, reliable, and accessible tests will emerge, helping clinicians make informed decisions that are tailored to each patient’s unique biological makeup.
Integrating Advanced Technologies for Better Predictive Power
One exciting aspect of future research is the integration of advanced technologies like artificial intelligence and machine learning. By analyzing vast amounts of data from patient registries, blood analyses, and genetic screenings, these technologies can help identify patterns that might be missed by traditional methods.
For instance, machine learning algorithms can be trained to recognize subtle variations in immune responses that signal an increased risk of side effects. These systems can then provide clinicians with real-time, data-driven insights to aid in treatment planning. Such integration of technology not only offers a way to simplify these “confusing bits” but also ensures that decisions are based on a comprehensive analysis of numerous factors.
Moreover, partnerships between technology companies and medical institutions will be critical. By pooling together expertise in bioinformatics, immunology, and clinical care, these collaborations can accelerate the development of predictive tools that are both reliable and easy to use in a busy clinical environment.
Integrating Alternative Medicine and Nutritional Insights in Cancer Care
While immunotherapy continues to revolutionize cancer treatment, it is important to acknowledge that a truly comprehensive approach to patient care may also incorporate elements of alternative medicine and nutrition. Holistic care, which addresses the entire well-being of a patient, might help reduce the severity of treatment side effects and improve overall outcomes.
Many patients explore complementary therapies to bolster their immune system, aiming to bolster their body’s resilience before, during, and after treatment. These practices include guided nutrition plans, herbal supplements, and mind-body therapies such as meditation and acupuncture.
Integrating nutritional guidance with biomarker research can offer additional clues about how the body reacts to cancer treatments. For example, certain dietary factors might influence the levels of inflammatory markers like TNF or interferon-gamma, potentially impacting a patient’s prognosis. Recognizing these connections can help healthcare providers design treatment regimens that are tailored not just to the biology of the tumor, but also to the overall health and well-being of the patient.
Holistic Approaches to Enhance Treatment Tolerance
For patients experiencing the nerve-racking unpredictability of side effects, complementary therapies may provide an extra layer of support. Some holistic strategies that could complement biomarker-driven approaches include:
- Personalized Nutrition Plans: Tailored diets that strengthen the immune system, reduce inflammation, and provide the necessary nutrients for overall health.
- Mind-Body Techniques: Practices such as meditation, yoga, and acupuncture can help reduce stress, which is known to impact immune responses significantly.
- Integrative Healthcare Consultations: Combining insights from conventional oncology with alternative medicine can help create balanced treatment plans aimed at enhancing quality of life.
While such complementary strategies are not a replacement for evidence-based medical treatments, they provide patients with additional options to help manage the overwhelming aspects of their journey. In a healthcare landscape that increasingly values personalized medicine, acknowledging and incorporating these alternative approaches may help achieve better overall results.
Conclusion: Charting a Safer and More Personalized Path in Cancer Treatment
The research emerging from UT Southwestern represents more than another scientific milestone; it embodies a vision for a future where cancer treatment is not only effective but also finely tuned to each patient’s individual risks. By identifying early immune signals that predict side effects from immunotherapy, this study opens up the possibility of customized treatment strategies that greatly enhance patient safety and outcomes.
While there remain several challenging bits—such as replicating findings across diverse populations and integrating complex biomarker data into daily clinical practice—the path forward is filled with promising potential. The key lies in continuing to piece together these fine details of a patient’s immune profile, so that clinicians can steer through the intricacies of treatment with greater confidence and precision.
As we look ahead, the alignment of advanced diagnostic tools, multi-disciplinary collaboration, and even complementary treatment approaches could signal a major shift in how immunotherapy is administered. By harnessing the power of biomarkers, we are moving closer to a reality where the question is not whether cancer side effects will occur, but how early interventions can minimize their impact and allow patients to focus on recovery and quality of life.
This integrated, forward-thinking approach to cancer care—blending cutting-edge research, technological innovation, and holistic treatment strategies—offers a glimpse into a future where every step in the treatment process is guided by precise, personalized insights. It is a future in which each patient’s fight against cancer is supported by tests and treatments that are as unique as they are, paving the way for safer and more successful outcomes.
In conclusion, the journey from identifying immune biomarkers to creating practical diagnostic tools is full of twists and turns, yet it is a journey that holds the promise of transforming lives. As we continue to figure a path through these confusing bits and nerve-racking challenges, one thing remains clear: individualized, data-driven treatment plans are no longer a distant dream, but an emerging reality set to redefine cancer care for future generations.
By continuing to take a closer look at the interplay between our immune systems and cancer treatments, and by embracing both modern scientific techniques and traditional supportive therapies, the medical community can forge a new era of treatment that is not only effective but also profoundly compassionate and personalized.
Key Takeaways for Patients and Healthcare Providers
To summarize, here are some important points derived from the study and the broader discussion of immunotherapy safety:
- Predictive Biomarkers: Early signs in the blood, such as elevated antibody-producing cells, heightened levels of interferon-gamma, and increased TNF signals, can indicate a higher risk of side effects.
- Personalized Treatment: Utilizing biomarker analysis can help tailor cancer treatments to each patient’s unique immune profile, potentially reducing harmful side effects.
- Holistic Approaches: Integrating personalized nutrition and complementary therapies may improve overall patient outcomes by enhancing the body’s natural capabilities to manage treatment-related stress.
- Collaborative Research: Robust institutional registries and multi-disciplinary collaboration are essential for validating these findings and developing reliable clinical applications.
- Future Innovations: Advanced technologies, including artificial intelligence and machine learning, are poised to improve our understanding and prediction of immunotherapy responses, bridging the gap between complex data and actual clinical practice.
These points highlight the exciting potential for a future where cancer treatments are both safer and more effective, driven by comprehensive, personalized insights that answer some of the most overwhelming challenges in modern oncology.
A Call to Action
The progress made thus far is promising, yet the road ahead requires continued innovation, rigorous research, and a willingness from the medical community to adapt to new protocols that emphasize personalized patient care. It is incumbent upon researchers, clinicians, and policymakers alike to support further studies that validate these early findings, expand the patient diversity of research cohorts, and streamline the integration of biomarker testing into clinical practice.
For patients and their families, this means advocating for access to comprehensive diagnostic tools and being informed about the latest advances that could impact treatment options. For healthcare providers, it means taking steps to stay updated on emerging technologies and being open to collaborative practices that pave the way for safer treatment pathways.
Ultimately, by working together to manage the confusing bits and fine points of cancer immunotherapy, we can aspire to create a healthcare environment where every patient is guided by data-driven insights, and where the promise of immunotherapy is fully realized with minimal risk of harmful side effects.
This journey, while challenging, is a collective effort—a mission that bridges cutting-edge science, mainstream clinical care, and compassionate patient support. By investing in this integrated approach, we not only enhance treatment outcomes but also build a future where personalized, precise, and predictable cancer care becomes the standard for all.
Originally Post From https://www.utsouthwestern.edu/newsroom/articles/year-2025/sept-immunotherapy-biomarkers.html
Read more about this topic at
The current status of biomarkers for predicting toxicity – PMC
Germline biomarkers predict toxicity to anti-PD1/PDL1 …