Tucatinib in the Modern Cancer Treatment Landscape
The recent findings from the HER2CLIMB-05 trial have sparked new conversations in the oncology community about the role of tucatinib in treating HER2-positive metastatic breast cancer. As we take a closer look at this new regimen, it becomes apparent that the trial results are not only statistically significant but also promising when it comes to reducing the risk of disease progression or death. In this opinion editorial, we will explore the many layers of this breakthrough, examine its benefits and potential challenges, and discuss how it may shape the future of cancer care. Our discussion considers both modern treatment approaches and the subtle details that make a significant difference in patient outcomes.
An Overview of Tucatinib’s Role in Treating HER2-Positive Metastatic Breast Cancer
In recent years, oncologists have been on the lookout for effective therapies that tackle the tricky parts of HER2-positive metastatic breast cancer. Tucatinib, in combination with two established agents, trastuzumab and pertuzumab, has been studied as part of first-line maintenance therapy. The primary aim of the HER2CLIMB-05 trial was to measure the improvement in progression-free survival (PFS) without compromising patient safety. The trial results suggest that adding tucatinib can further lower the risk of disease progression or death in this challenging setting.
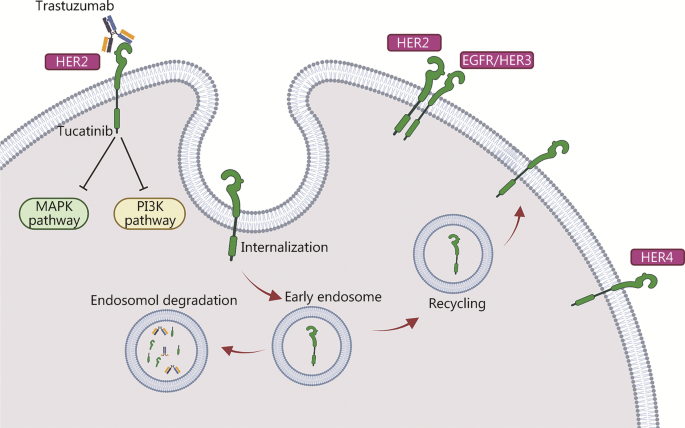
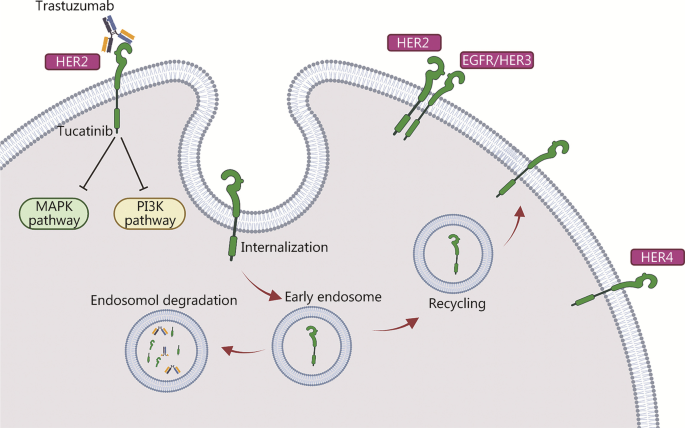
The mechanism of action of tucatinib is particularly noteworthy. It specifically targets the HER2 receptor and has shown a complementary effect when used alongside trastuzumab and pertuzumab. This combination is designed to create a multi-faceted blockade against cancer growth while keeping side effects within an acceptable range. Although the details of drug interactions can sometimes be confusing bits, the established safety profile of each agent provides comfort to both clinicians and patients.
The Importance of First-Line Maintenance Therapy: Benefits and Considerations
Adopting tucatinib in the first-line maintenance setting offers a strategic advantage. Traditionally, many new agents are reserved for later-line treatments after initial therapies have failed. However, using tucatinib early on provides the opportunity to combat the disease right from the start of the maintenance phase. This approach is seen as both essential and super important in shifting treatment paradigms.
When we consider the benefits of first-line maintenance therapy, several key points emerge:
- Earlier intervention may prolong progression-free intervals.
- Patients could experience a delay in the onset of secondary treatments.
- The well-known safety profile reduces the risk of unexpected adverse events.
These advantages are balanced against the need for thorough patient evaluation to ensure that the treatment is appropriate for each individual case. There are still some tangled issues regarding patient selection, dosing strategies, and long-term tolerability that need to be considered. Nevertheless, the available data show a clear path toward expanding our treatment menu for HER2-positive patients.
Decoding the Safety Profile: Side Effects and Their Management
One of the fundamental aspects that practitioners scrutinize is the safety profile provided by the tucatinib-based regimen. As with many cancer treatments, side effects are inevitable, but the management of these adverse effects is key to patient success. In the HER2CLIMB-05 trial, the safety profile of tucatinib was consistent with previous findings, thus giving clinicians confidence in its controlled application.
Common adverse effects observed in the trial included:
- Diarrhea
- Vomiting
- Nausea
- Abdominal pain
- Seizure (in a small percentage of patients)
More severe warnings associated with the treatment involve risks such as severe diarrhea, dehydration, hypotension, acute kidney injury, and even potential embryo-fetal toxicities. Such side effects can be intimidating for patients, but they are manageable when clinicians maintain close monitoring and employ prophylactic measures where necessary.
A brief table summarizes the notable adverse events and the typical responses in clinical practice:
| Adverse Event | Common Response |
|---|---|
| Diarrhea | Antidiarrheal agents, hydration therapy |
| Hepatotoxicity | Liver enzyme monitoring, dose adjustment |
| Dehydration | IV fluids, electrolyte management |
| Acute Kidney Injury | Renal function monitoring, dose modifications |
Understanding and managing these side effects is especially important because it helps ensure that the benefits of the treatment outweigh its potential risks. Clinicians and patients alike are encouraged to engage closely to figure a path through the complicated pieces of treatment management to arrive at the best possible outcome.
Combination Therapy: Tucatinib with Trastuzumab and Pertuzumab
Combination therapy is not new in the treatment of HER2-positive diseases, and the integration of tucatinib with trastuzumab and pertuzumab marks another step in this evolutionary process. The science behind combining these agents is built on the idea that using drugs with complementary mechanisms can overcome some of the tricky parts that each drug might not address on its own.
Here is a closer look at the three-drug regimen:
- Tucatinib: A small molecule inhibitor that specifically targets the HER2 receptor, offering a focused approach to hinder cancer proliferation.
- Trastuzumab: A monoclonal antibody that binds to the HER2 receptor, flagging the cancer cells for the immune system.
- Pertuzumab: Another monoclonal antibody that disrupts HER2 receptor dimerization, preventing the activation of pathways that lead to tumor growth.
This orchestrated effort ensures that multiple attacking angles are deployed against the tumor cells. When used together, these agents provide a robust blockade against disease progression, while their safety profiles continue to support their combined use, even as some of the fine shades in their mechanisms of action remain the subject of ongoing research.
Challenges in Managing HER2-Positive Cancer: Fine Points and Hidden Complexities
Despite the promising data, the treatment of HER2-positive breast cancer remains loaded with issues and tricky parts. Among these are the responsibilities that physicians face in managing side effects, monitoring for potential drug interactions, and maintaining patient quality of life. Although the trial results offer a ray of hope, they also remind us that every new treatment comes with its own set of challenges.
Some of the areas that require careful consideration include:
- Patient Selection: Not all patients will benefit equally from the addition of tucatinib. Selecting the right candidate is a super important task that requires a detailed medical history and careful evaluation of previous treatments.
- Dosing Adjustments: The risk of adverse events such as hepatotoxicity and diarrhea means that clinicians must be ready to adjust doses on a case-by-case basis.
- Monitoring and Support: Enhanced monitoring protocols must be in place to work through the subtle details involved in continuous patient care, ensuring that side effects are identified early and managed appropriately.
In many ways, these challenges are similar to trying to find your way through a maze filled with unforeseen turns. However, with precise patient feedback and diligent monitoring, these complications can be addressed in a timely manner, giving hope to those who have been battling this nerve-racking disease for too long.
Comparative Insights: Later-Line vs. First-Line Treatment Settings
Historically, tucatinib was approved for use in later-line settings, where it was reserved for patients who had already undergone one or more lines of HER2-based treatments. The transition of tucatinib into the first-line maintenance arena is therefore significant, as it may benefit a broader segment of patients earlier in their treatment journey.
When comparing the later-line and first-line settings, several factors come to light:
- Efficacy Improvements: Early intervention with tucatinib appears to further delay disease progression, potentially extending patients’ life quality and expectancy.
- Safety and Tolerability: By applying a well-established dosage used in later settings, clinicians can leverage their existing experience, thereby reducing some of the scary uncertainties associated with new treatment combinations.
- Quality of Life: Delaying the occurrence of severe relapse not only improves clinical outcomes but also enhances the overall well-being of patients. The goal is not merely to extend life but also to maintain a level of quality that allows patients to enjoy it.
In essence, moving tucatinib from a later-line to a first-line setting signifies a shift toward early optimization of cancer control, which could prove transformative for both patients and healthcare providers.
Clinical Trial Details: A Closer Look at the HER2CLIMB-05 Study
The HER2CLIMB-05 trial has provided key insights into the benefits and limitations of using tucatinib as part of a maintenance therapy regimen. Conducted as a double-blind phase 3 study, the trial enrolled patients with HER2-positive metastatic breast cancer who had already completed taxane-based induction therapy. The trial’s design involved randomizing patients to receive either tucatinib alongside trastuzumab and pertuzumab or a placebo with the same maintenance partners.
This study design was devised to answer several important questions:
- Does the addition of tucatinib significantly extend progression-free survival (PFS) compared to placebo?
- Are the side effects of the combination manageable within established parameters?
- How does this new regimen affect the overall treatment flow for patients with HER2-positive metastatic breast cancer?
This trial not only met its primary endpoint of PFS as assessed by investigators but also demonstrated that the tucatinib regimen was generally well-tolerated. The data suggest that the treatment adds a valuable layer of defense against disease progression, without exposing patients to an unmanageable side-effect load. Such clinical evidence provides a strong foundation for rethinking our treatment strategies and for further research into refining patient care practices.
Evaluating Quality of Life: Beyond Clinical Efficacy
Beyond merely prolonging progression-free intervals, a key consideration for any cancer treatment is its impact on the patient’s overall quality of life. The HER2CLIMB-05 trial also looked into secondary endpoints such as overall survival and health-related quality of life factors. Although these measures involve some of the small distinctions that only long-term data can fully reveal, initial trends seem encouraging.
Quality of life measurements are critical because they help medical professionals get into the nitty-gritty of patient care. In clinical practice, enhanced quality of life translates to:
- Fewer hospitalizations and emergency interventions
- Better physical and emotional well-being
- Greater opportunities to maintain daily routines and professional responsibilities
Addressing these fine points is essential, as a treatment that merely extends life without preserving its quality may not always be acceptable to patients and their families. The integration of tucatinib in the early maintenance stage suggests that these quality of life considerations are being taken seriously as part of a comprehensive treatment strategy.
Integrating Innovative Therapies into Current Oncology Practices
The adoption of tucatinib as a first-line maintenance therapy reflects the evolving face of modern oncology. The treatment landscape for HER2-positive metastatic breast cancer is increasingly being shaped by innovative therapies that combine established agents with new ones to counter the disease effectively. However, successfully steering through these changes means understanding both the benefits and the twists and turns that come with them.
When integrating new therapies into clinical practice, the following steps are often crucial:
- Comprehensive Training: Healthcare providers must receive ongoing education about the latest research findings and treatment protocols.
- Multidisciplinary Collaboration: Oncologists, nurses, pharmacists, and support care teams should work in tandem to ensure treatment regimens are followed accurately.
- Data-Driven Adjustments: Real-world evidence and clinical trial data need to be periodically reviewed so that adjustments and improvements can be made.
The inclusion of tables, detailed bullet lists, and regular updates in clinical practice guidelines all help create a framework that allows for smoother implementation of new therapies. This collaborative, data-driven approach not only improves patient outcomes but also ensures that everyone in the treatment team is working together to sort out any challenging issues as they arise.
Reflections on the Future of HER2-Positive Cancer Treatment
As we look to the future, the implications of the HER2CLIMB-05 trial extend far beyond the immediate study findings. By successfully incorporating tucatinib into the first-line maintenance setting, oncologists have opened the door to a broader understanding of how to effectively combat HER2-positive metastatic breast cancer. While there remain some confusing bits and nerve-racking uncertainties, the overall tone is one of cautious optimism.
Key takeaways for the future include:
- Early Intervention: Starting treatment early, with a regimen that balances efficacy and safety, may become a new standard of care for patients with aggressive HER2-positive cancers.
- Patient-Centered Approaches: Future research must continue to prioritize quality of life alongside traditional clinical endpoints, ensuring that treatments are not only effective but also sustainable over the long term.
- Continuous Innovation: As newer agents and individualized therapies are developed, ongoing research will be essential to streamline treatment workflows and adapt to each patient’s unique needs.
The path forward in oncology is likely to be full of tricky parts and tangled issues. Nonetheless, with each new study and each advance in treatment, we move closer to a future where managing HER2-positive metastatic breast cancer becomes less intimidating and more predictable. As clinicians and researchers get into the fine points of these emerging treatments, the overall goal remains clear: to extend survival while maintaining the highest possible quality of life for every patient.
Final Thoughts: A Collective Step Forward in Cancer Care
In summary, the introduction of tucatinib as part of first-line maintenance therapy for HER2-positive metastatic breast cancer marks a significant milestone in oncology. The promising results of the HER2CLIMB-05 trial provide both clinicians and patients with an additional tool to fight a disease that, until now, has been riddled with challenges and nerve-racking obstacles.
As we reflect on the evolution of treatment options, it is clear that the combined use of tucatinib, trastuzumab, and pertuzumab represents not only a scientific advancement but also a fundamental shift toward a more proactive approach in cancer care. This strategy allows us to dig into the subtle details of tumor biology and treatment response, applying a more comprehensive and patient-oriented management plan.
Of course, no new treatment is free from concerns. Careful patient selection, diligent monitoring, and a commitment to managing side effects remain as critical as ever. By working together across the multidisciplinary team, healthcare providers can steer through the complicated pieces of treatment planning to ensure the best possible outcomes.
Ultimately, the HER2CLIMB-05 trial is more than just a study—it is a reflection of the ongoing pursuit of excellence in cancer treatment. As we continue to explore and embrace innovative therapies, we pave the way for better, more effective, and less intimidating treatment strategies that can truly make a difference in the lives of patients.
In a field where every little twist and turn matters, the role of tucatinib in improving progression-free survival stands out as a meaningful advancement. While challenges remain and further studies will be needed to fully understand the long-term impacts, current evidence offers a hopeful glimpse into the bright future of targeted oncology therapies.
Looking ahead, the cancer care community must remain committed to forging collaborative paths, sharing real-world experiences, and continuously updating treatment protocols to reflect the latest scientific insights. For many patients facing the overwhelming reality of metastatic breast cancer, these advances symbolize not only new medical options but also renewed hope and improved quality of life.
In conclusion, the integration of tucatinib into first-line maintenance therapy for HER2-positive metastatic breast cancer is a testament to the power of innovative research and the relentless pursuit of improved patient care. While the journey through this complex treatment landscape may involve tricky parts and tangled issues, each step forward brings us closer to a future where effective, personalized cancer therapy is a reality for everyone faced with this formidable challenge.
Originally Post From https://www.cancernetwork.com/view/tucatinib-regimen-improves-pfs-in-her2-metastatic-breast-cancer
Read more about this topic at
ENHERTU® Plus Pertuzumab Granted Breakthrough …
FDA Grants Breakthrough Therapy Designation to Frontline …